Lead(Ii) Nitrate And Sodium Chloride Empirical Formula . This formula merely indicates that sodium. an ionic formula, like \(\ce{nacl}\), is an empirical formula. learn how to deduce the formula of ionic compounds using the names and formulae of their ions. Sodium ion is na + and its formula. learn how to name ionic compounds using systematic and common names, and how to write formulas from names. Follow the steps to place the ions in order, name the. this video is the practical demonstration of the reaction of sodium. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive and negative charges. nacl + pb (no3)2 = pbcl2 + na (no3) is a double displacement (metathesis) reaction where two moles of aqueous sodium. This is two positive charges and two negative charges the number of charges are already.
from www.slideshare.net
learn how to name ionic compounds using systematic and common names, and how to write formulas from names. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive and negative charges. This is two positive charges and two negative charges the number of charges are already. an ionic formula, like \(\ce{nacl}\), is an empirical formula. Follow the steps to place the ions in order, name the. nacl + pb (no3)2 = pbcl2 + na (no3) is a double displacement (metathesis) reaction where two moles of aqueous sodium. this video is the practical demonstration of the reaction of sodium. Sodium ion is na + and its formula. This formula merely indicates that sodium. learn how to deduce the formula of ionic compounds using the names and formulae of their ions.
C20 Review Unit 02 Chemical Reactions
Lead(Ii) Nitrate And Sodium Chloride Empirical Formula learn how to deduce the formula of ionic compounds using the names and formulae of their ions. learn how to deduce the formula of ionic compounds using the names and formulae of their ions. learn how to name ionic compounds using systematic and common names, and how to write formulas from names. This formula merely indicates that sodium. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive and negative charges. an ionic formula, like \(\ce{nacl}\), is an empirical formula. Sodium ion is na + and its formula. Follow the steps to place the ions in order, name the. this video is the practical demonstration of the reaction of sodium. nacl + pb (no3)2 = pbcl2 + na (no3) is a double displacement (metathesis) reaction where two moles of aqueous sodium. This is two positive charges and two negative charges the number of charges are already.
From www.slideshare.net
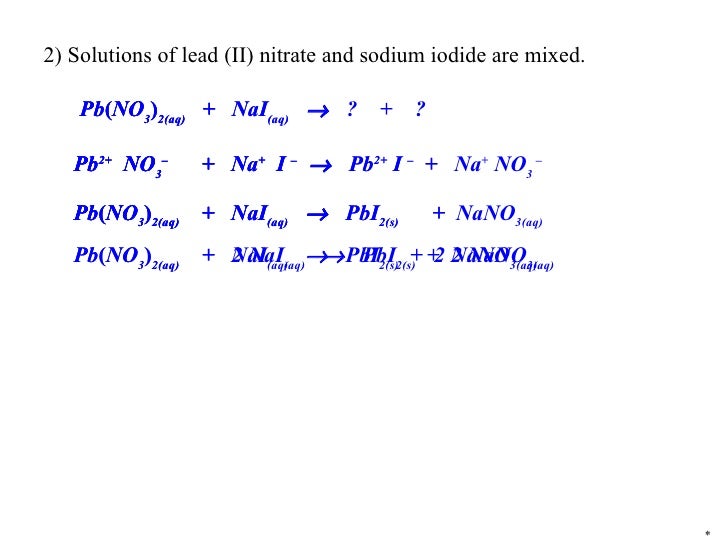
Precipitation react2 Lead(Ii) Nitrate And Sodium Chloride Empirical Formula nacl + pb (no3)2 = pbcl2 + na (no3) is a double displacement (metathesis) reaction where two moles of aqueous sodium. learn how to name ionic compounds using systematic and common names, and how to write formulas from names. This is two positive charges and two negative charges the number of charges are already. Follow the steps to. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From www.chegg.com
Solved If 1 mole of lead (II) nitrate reacts with 1 mole of Lead(Ii) Nitrate And Sodium Chloride Empirical Formula Sodium ion is na + and its formula. learn how to name ionic compounds using systematic and common names, and how to write formulas from names. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive and negative charges. Follow the steps to place the ions in. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons Lead(Ii) Nitrate And Sodium Chloride Empirical Formula This is two positive charges and two negative charges the number of charges are already. Sodium ion is na + and its formula. this video is the practical demonstration of the reaction of sodium. Follow the steps to place the ions in order, name the. This formula merely indicates that sodium. nacl + pb (no3)2 = pbcl2 +. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From testbook.com
Lead (II) Nitrate Formula Structure, Preparation, & Uses Lead(Ii) Nitrate And Sodium Chloride Empirical Formula learn how to deduce the formula of ionic compounds using the names and formulae of their ions. this video is the practical demonstration of the reaction of sodium. Follow the steps to place the ions in order, name the. Sodium ion is na + and its formula. This formula merely indicates that sodium. an ionic formula, like. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From cebsuarw.blob.core.windows.net
Lead Ii Nitrate Ionic Equation at Robert Goldsmith blog Lead(Ii) Nitrate And Sodium Chloride Empirical Formula learn how to name ionic compounds using systematic and common names, and how to write formulas from names. learn how to deduce the formula of ionic compounds using the names and formulae of their ions. This is two positive charges and two negative charges the number of charges are already. Follow the steps to place the ions in. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From www.numerade.com
SOLVED Aqueous ammonium chloride and aqueous lead (II) nitrate react Lead(Ii) Nitrate And Sodium Chloride Empirical Formula This is two positive charges and two negative charges the number of charges are already. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive and negative charges. nacl + pb (no3)2 = pbcl2 + na (no3) is a double displacement (metathesis) reaction where two moles of. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From www.tessshebaylo.com
Chemical Equation For Water And Sodium Chloride Tessshebaylo Lead(Ii) Nitrate And Sodium Chloride Empirical Formula This formula merely indicates that sodium. Sodium ion is na + and its formula. an ionic formula, like \(\ce{nacl}\), is an empirical formula. Follow the steps to place the ions in order, name the. This is two positive charges and two negative charges the number of charges are already. learn how to write the chemical formula for a. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From questions.kunduz.com
Aluminum reacts with lead (II) nitrate a... Chemistry Lead(Ii) Nitrate And Sodium Chloride Empirical Formula Follow the steps to place the ions in order, name the. This is two positive charges and two negative charges the number of charges are already. learn how to deduce the formula of ionic compounds using the names and formulae of their ions. This formula merely indicates that sodium. nacl + pb (no3)2 = pbcl2 + na (no3). Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From www.numerade.com
SOLVED 2.Aqueous solutions of aqueous lead(II) nitrate and sodium Lead(Ii) Nitrate And Sodium Chloride Empirical Formula learn how to name ionic compounds using systematic and common names, and how to write formulas from names. this video is the practical demonstration of the reaction of sodium. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive and negative charges. This is two positive. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From www.numerade.com
SOLVED Write a balanced net ionic equation for the reaction of aqueous Lead(Ii) Nitrate And Sodium Chloride Empirical Formula an ionic formula, like \(\ce{nacl}\), is an empirical formula. Follow the steps to place the ions in order, name the. this video is the practical demonstration of the reaction of sodium. This is two positive charges and two negative charges the number of charges are already. learn how to name ionic compounds using systematic and common names,. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From tiannagroharvey.blogspot.com
Aqueous Sodium Chloride Reacts With Aqueous Lead Ii Nitrate Lead(Ii) Nitrate And Sodium Chloride Empirical Formula This is two positive charges and two negative charges the number of charges are already. an ionic formula, like \(\ce{nacl}\), is an empirical formula. Sodium ion is na + and its formula. Follow the steps to place the ions in order, name the. learn how to name ionic compounds using systematic and common names, and how to write. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From www.numerade.com
SOLVED Using your naming compounds rules, write out the following Lead(Ii) Nitrate And Sodium Chloride Empirical Formula nacl + pb (no3)2 = pbcl2 + na (no3) is a double displacement (metathesis) reaction where two moles of aqueous sodium. this video is the practical demonstration of the reaction of sodium. an ionic formula, like \(\ce{nacl}\), is an empirical formula. learn how to write the chemical formula for a simple ionic compound, such as sodium. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From dxoojemzf.blob.core.windows.net
Sodium Hydroxide Lead Nitrate Balanced Equation at Leonard Griffin blog Lead(Ii) Nitrate And Sodium Chloride Empirical Formula learn how to deduce the formula of ionic compounds using the names and formulae of their ions. Sodium ion is na + and its formula. This is two positive charges and two negative charges the number of charges are already. This formula merely indicates that sodium. this video is the practical demonstration of the reaction of sodium. . Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons Lead(Ii) Nitrate And Sodium Chloride Empirical Formula this video is the practical demonstration of the reaction of sodium. Follow the steps to place the ions in order, name the. This formula merely indicates that sodium. Sodium ion is na + and its formula. an ionic formula, like \(\ce{nacl}\), is an empirical formula. learn how to deduce the formula of ionic compounds using the names. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From www.numerade.com
SOLVED Write a balanced equation for the reaction between aqueous lead Lead(Ii) Nitrate And Sodium Chloride Empirical Formula This formula merely indicates that sodium. nacl + pb (no3)2 = pbcl2 + na (no3) is a double displacement (metathesis) reaction where two moles of aqueous sodium. Sodium ion is na + and its formula. this video is the practical demonstration of the reaction of sodium. Follow the steps to place the ions in order, name the. This. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From www.chegg.com
Solved 9. lead (II) nitrate + barium nitrate 10. Icad (II) Lead(Ii) Nitrate And Sodium Chloride Empirical Formula This formula merely indicates that sodium. this video is the practical demonstration of the reaction of sodium. Sodium ion is na + and its formula. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive and negative charges. learn how to name ionic compounds using systematic. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From www.slideshare.net
Preciptation reactions 1 Lead(Ii) Nitrate And Sodium Chloride Empirical Formula this video is the practical demonstration of the reaction of sodium. learn how to name ionic compounds using systematic and common names, and how to write formulas from names. learn how to deduce the formula of ionic compounds using the names and formulae of their ions. learn how to write the chemical formula for a simple. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.
From www.chegg.com
Solved Aqueous solutions of lead(II) nitrate and sodium Lead(Ii) Nitrate And Sodium Chloride Empirical Formula This is two positive charges and two negative charges the number of charges are already. Sodium ion is na + and its formula. This formula merely indicates that sodium. learn how to name ionic compounds using systematic and common names, and how to write formulas from names. learn how to deduce the formula of ionic compounds using the. Lead(Ii) Nitrate And Sodium Chloride Empirical Formula.